 NAFDAC Product Registration for what? Like I said in my article for how to process and obtain Form M in Nigeria, every single shipment into Nigeria is meant to have been subjected to the Form M requirement in Nigeria. Thus, what this means is that such shipment should have been certified by the Standards Organisation of Nigeria (SON). Also, the SON Product Certificate and the SONCAP certificate issued. However, certain defined goods and items are not regulated by the Standards Organisation of Nigeria. Consequently they are exempt from the SONCAP requirements in Nigeria.
NAFDAC Product Registration for what? Like I said in my article for how to process and obtain Form M in Nigeria, every single shipment into Nigeria is meant to have been subjected to the Form M requirement in Nigeria. Thus, what this means is that such shipment should have been certified by the Standards Organisation of Nigeria (SON). Also, the SON Product Certificate and the SONCAP certificate issued. However, certain defined goods and items are not regulated by the Standards Organisation of Nigeria. Consequently they are exempt from the SONCAP requirements in Nigeria.
Table of Contents
ToggleMany of those SONCAP exempted commodities are taken care of by the National Agency for Food and Drugs Administration and Control (NAFDAC). NAFDAC is an agency of the Federal Ministry of Health in Nigeria. If you are familiar with the Nigerian people, the mention of NAFDAC could probably remind you of the late icon Dora Akunyili, whose works brought fame to NAFDAC.
The purpose of this article is not to eulogise the late Dora Akunyili, but to give importers the guide needed to process and obtain Permit to import industrial and laboratory chemicals.
NAFDAC PRODUCT REGISTRATION: ITEMS EXEMPT FROM SONCAP REQUIREMENTS IN NIGERIA
- Food products
- Drugs (medicines not the other kind of drugs for wrong use)
- Chemicals used as raw materials
- Military wares and equipment
- Used products; Read Here: CUSTOMS CLEARANCE: NESREA CLEARANCE PERMIT
- Products included in the prohibited lists
- Medicals except equipment and machines
- Branded products with minimal quantities, and meant for gift items or promotional items may be exempt from SONCAP requirements in Nigeria.
NAFDAC PRODUCT REGISTRATION: PERMIT REQUIREMENTS
- You must be a registered company in Nigeria, with CAC documents
- Products should have the Material Safety Data Sheet – MSDS of the chemical
- You must have employed a technical officer with a minimum of OND qualification in chemistry or related field of study
- A non-refundable processing fee of seventy nine thousand naira (NGN79,000), for the first page of not more than 25 items, additional pages cost thirty three thousand seven hundred and fifty thousand naira (NGN33,750)
- You must ensure a verifiable warehouse for storage is in place for inspected
- Evidence of registration by NAFDAC for companies manufacturing the agency’s regulated product.
- Companies applying for chemicals whose use require other agencies approval like DPR Permit, must attach such certificates or permit
- Processing of permit could take one or two weeks
- Do not import items before requesting for Permit. Otherwise, fine of NGN135,000.00 applies.
In this article, we will focus only on electronic application of NAFDAC import Permit for importation of industrial and laboratory chemicals. Kindly check the below link for the importation of other products.
Read Also: SONCAP IMPORT PERMIT: EXEMPTED PRODUCTS NIGERIA
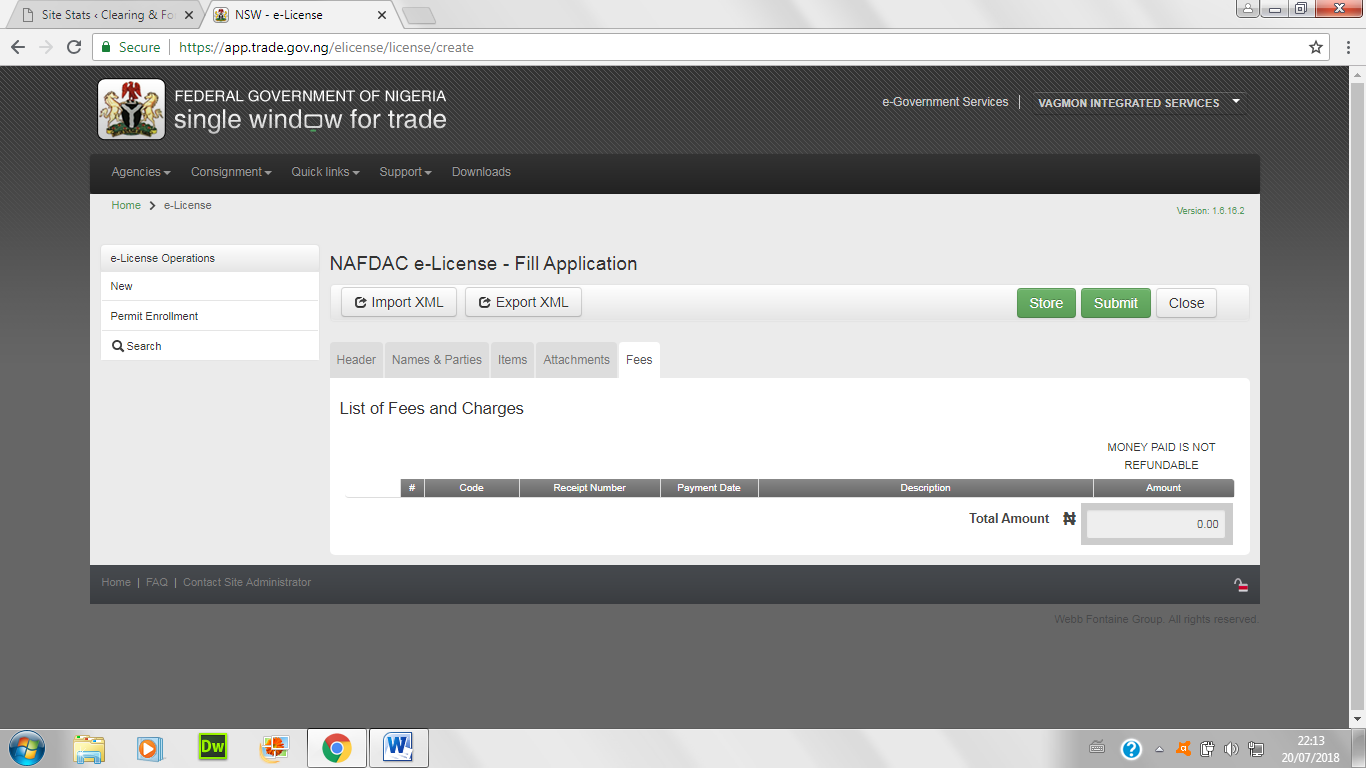
NAFDAC PRODUCT REGISTRATION: APPLICATION FOR NAFDAC E-LICENCE IMPORT PERMIT
- Go to here, login with your Tax Identification Number (TIN) and username
- Under the menu for agency, choose NAFDAC
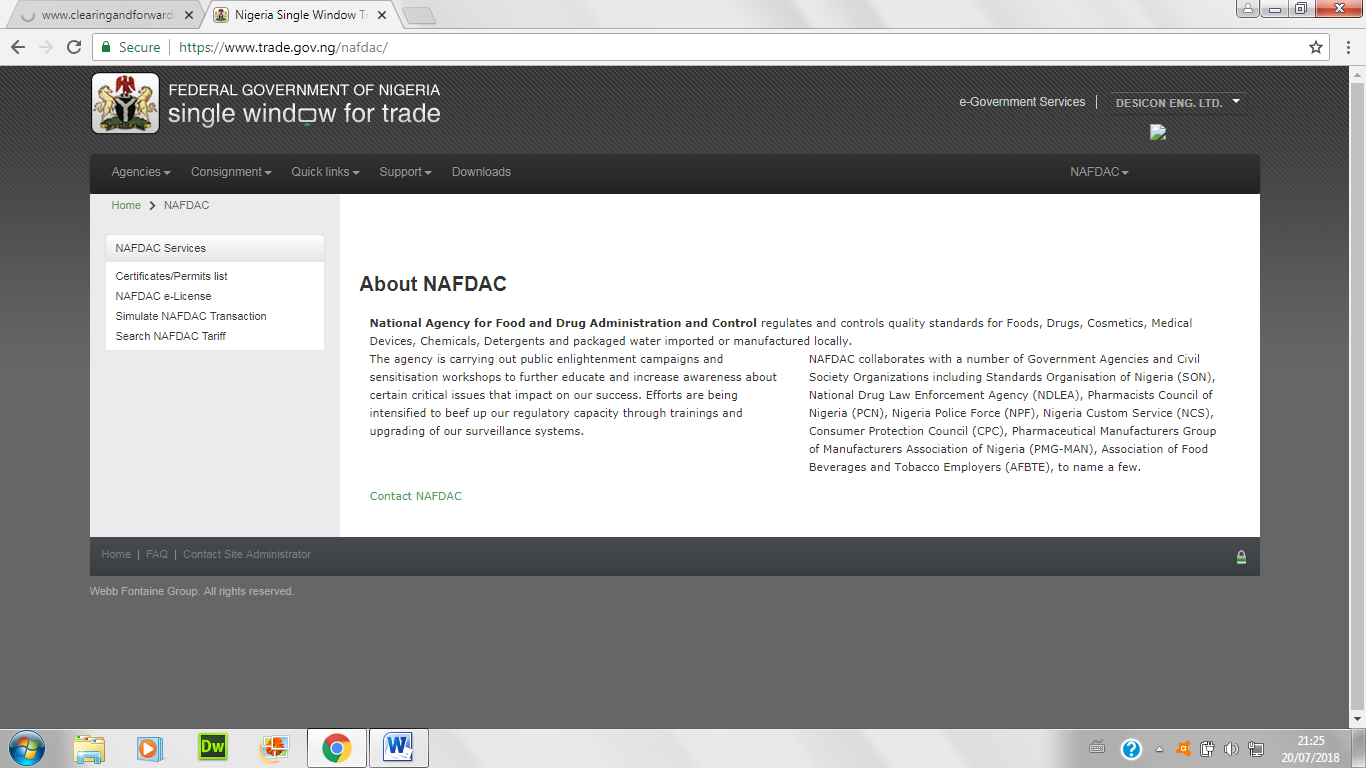
- Choose the NAFDAC e-licence; and fill application and complete the first page below:
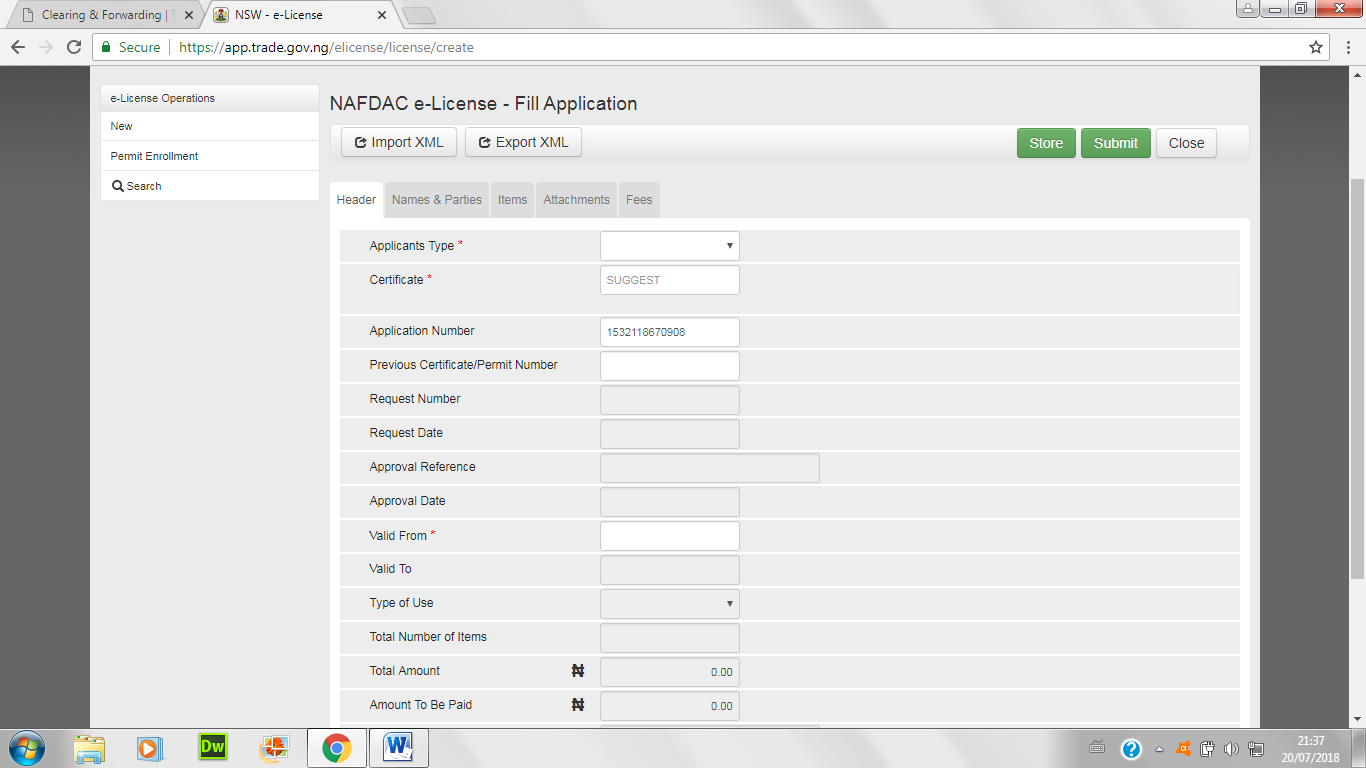
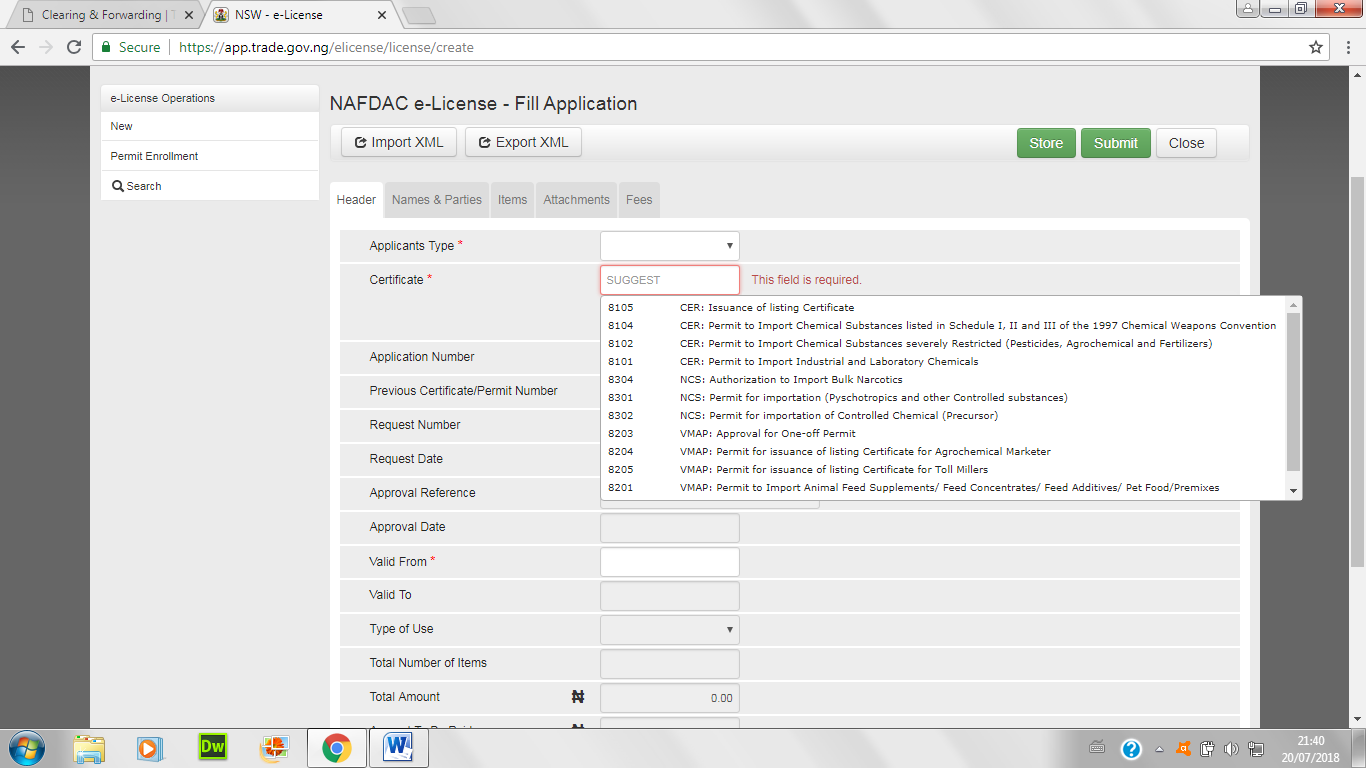
- Also, choose from the list the kind of permit sought after
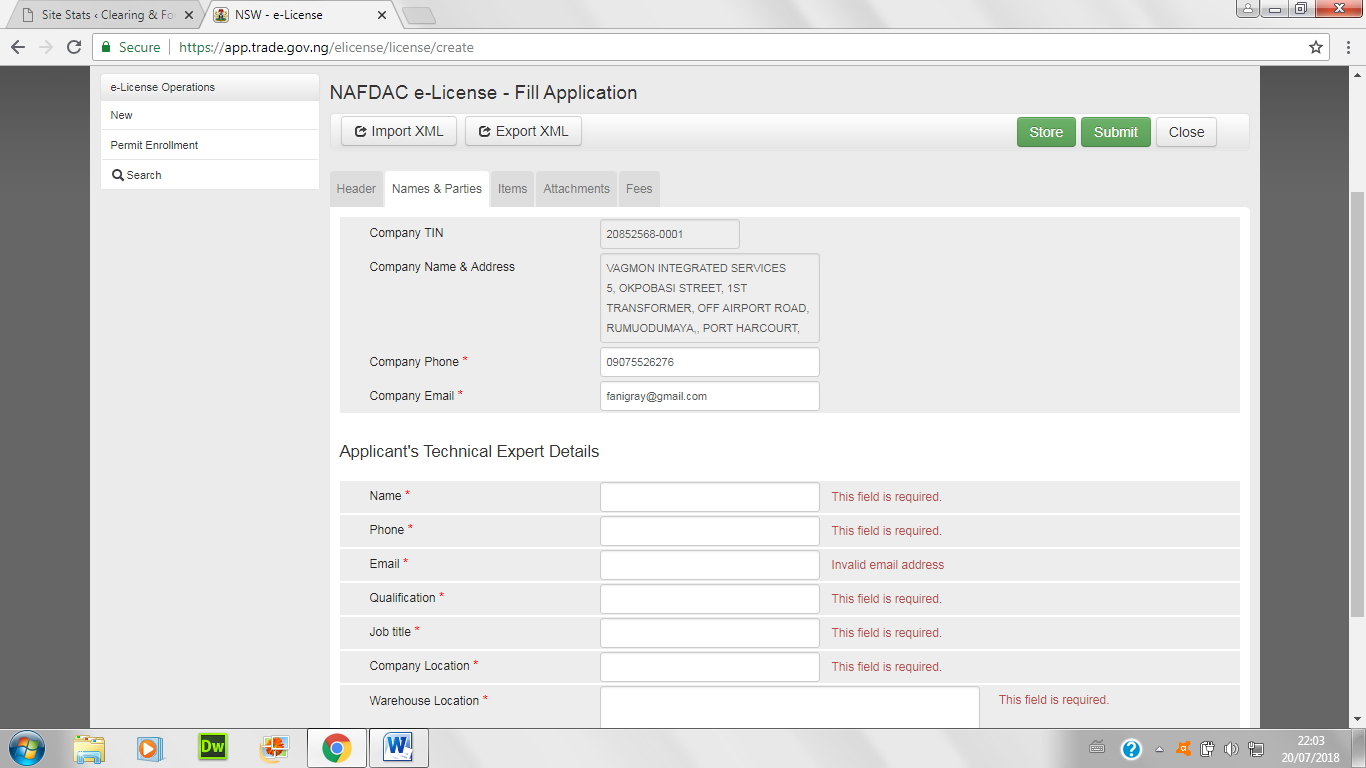
- Fill in the information of the technical officer detail and the warehouse location below:
- Enter items of import below; either convert the excel sheet to xml by choosing xml data in the save button, or type items one-by-one.
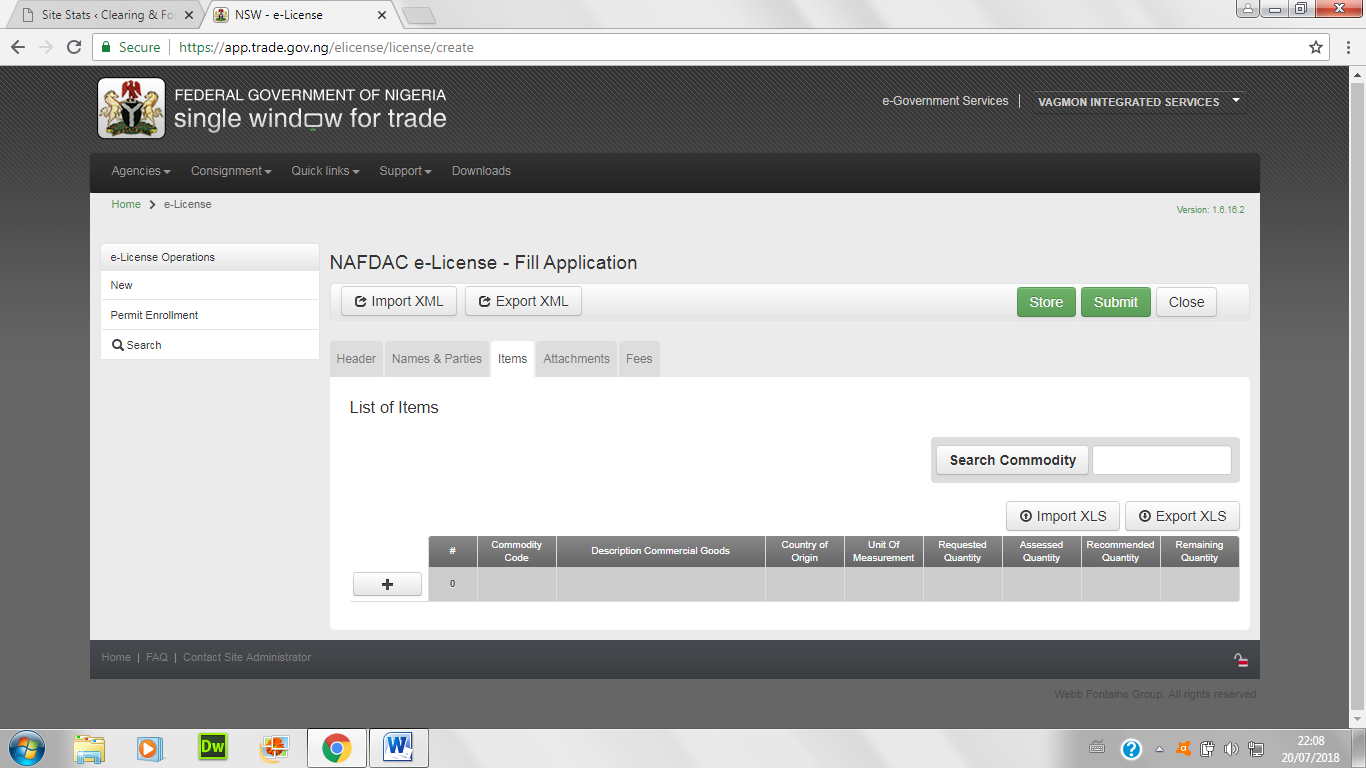
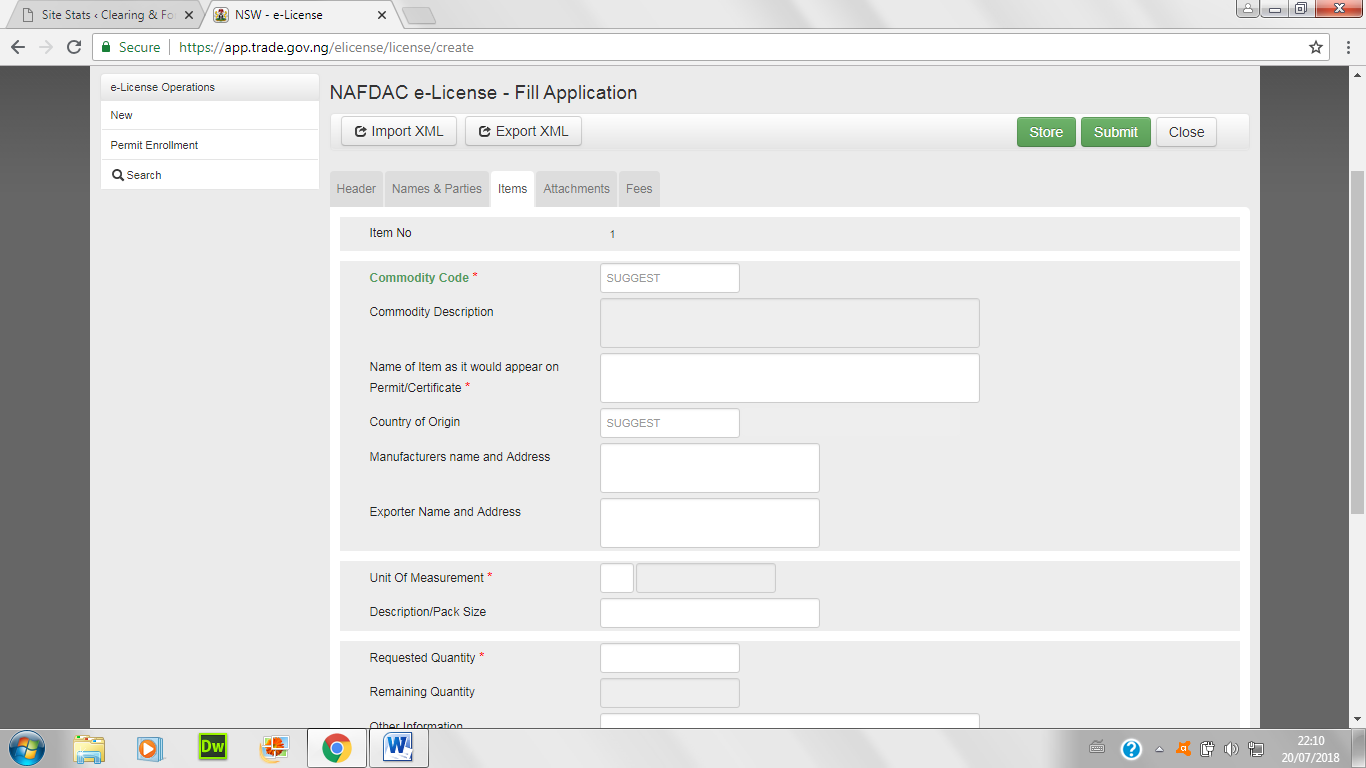
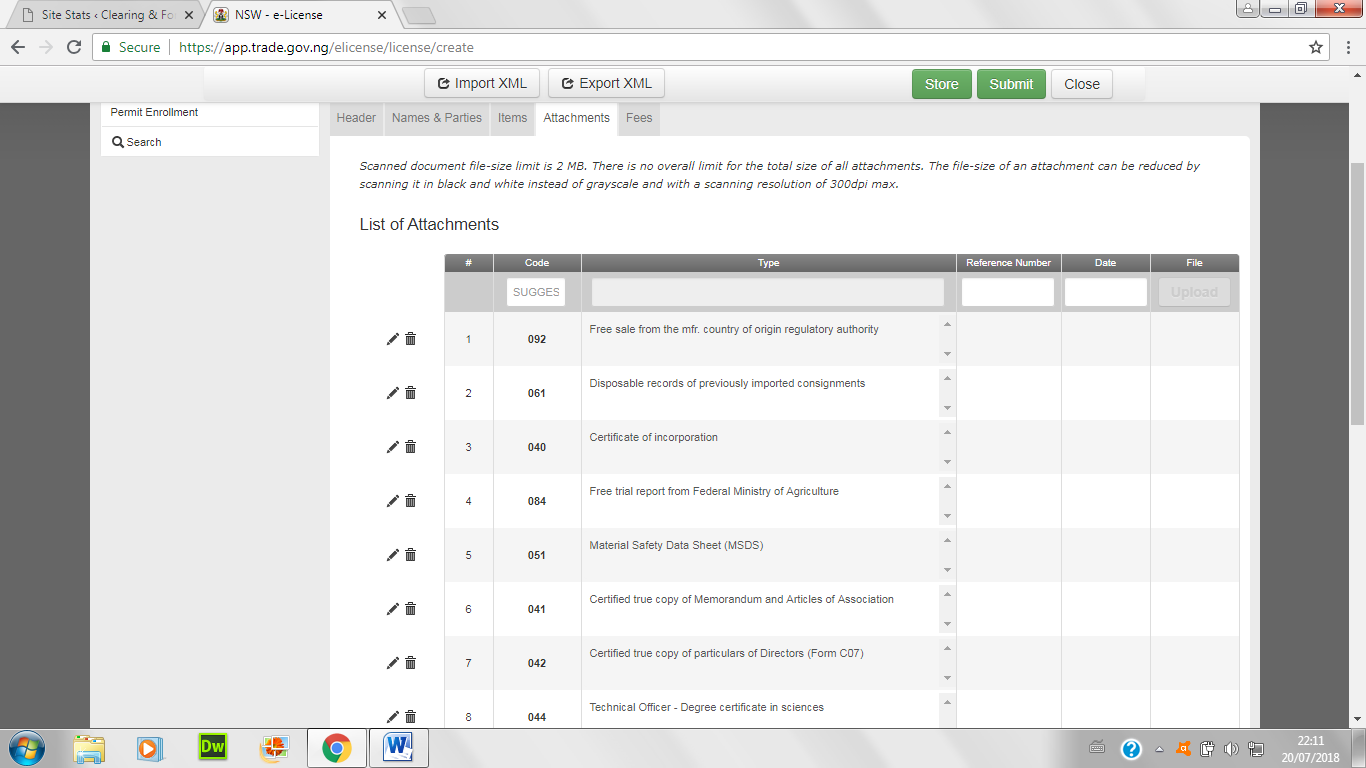
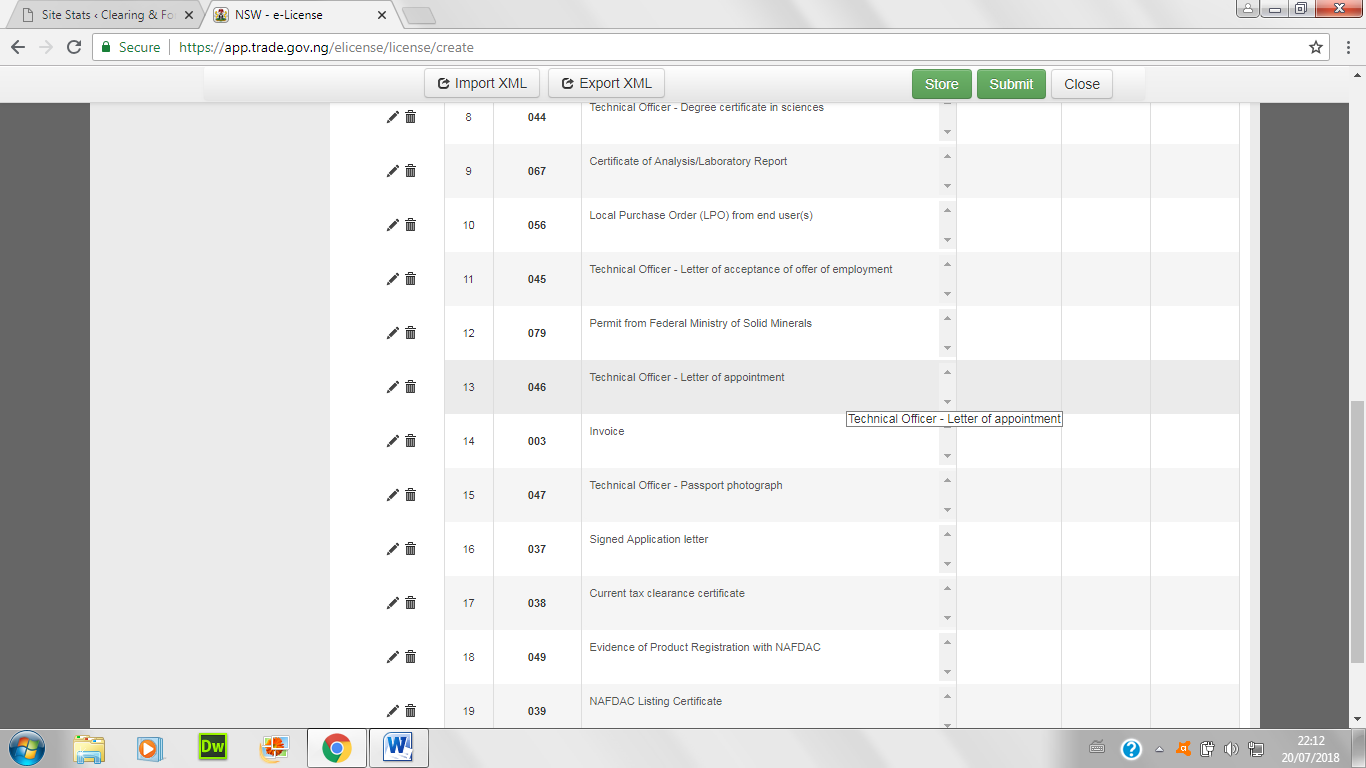
- Attach all necessary certificates and papers below
- Once all items are entered, the bill gets generated. re-enter the amount below and then submit the application.
In Conclusion:
Form M requirement in Nigeria means that every importer must first of all obtain SON Product certification, since this is the foremost requirement in Form M processing. However, certain commodities are exempt from the SONCAP requirements in Nigeria. This article has dealt with how to obtain permit to import industrial and lab chemicals using the electronic option.
Watch also:

